Corrosion
Cathodic Protection
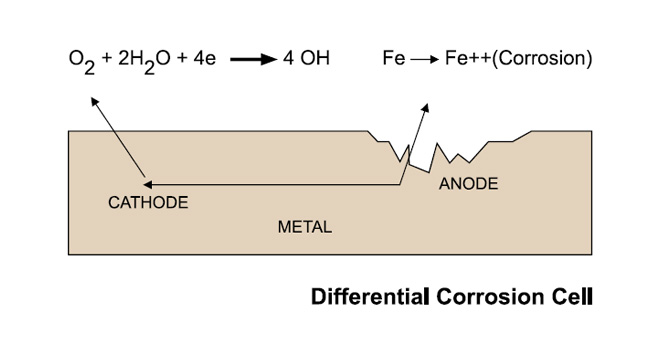
When two dissimilar metals are connected in an electrolyte such as sea-water, a corrosion cell is formed in which one metal becomes an anode and suffers corrosion while the other metal becomes the cathode and is preserved.
Anodic and cathodic areas exist on the surface of all steel structures due to slight variations in materials composition, local stresses, differences in coating condition and availability of oxygen.
On a bare steel plate in sea-water, these areas form on what is apparently a uniform surface. Since corrosion will result in metal loss, roughening and wastage will occur on an uncoated plate. Where an imperfect coating exists, corrosion will take the form of accelerated pitting at the location of the bare spots and eventual breakdown of the coating.
Whatever the circumstances, in the absence of cathodic protection, corrosion will eventually occur.
The principle of cathodic protection involves the introduction into the electrical circuit of a metal that is more electro-negative than the existing anodic and cathodic areas.
This additional metal becomes the anode and will corrode while providing current to the metal it is protecting, thereby overcoming the local anodic areas and making them cathodic.
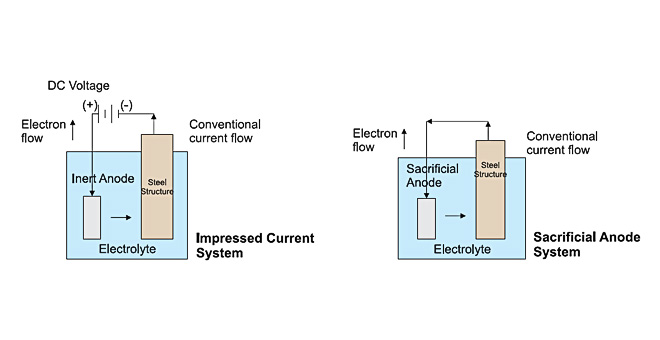
This classic cathodic protection solution to a galvanic corrosion problem is to introduce an anode in a suitable alloy of magnesium, aluminium or zinc which will suffer corrosion and so be ‘sacrificed’ in protecting the cathode. This approach is used in Sacrificial Systems.
As an alternative solution, the protective current needed to make the structure fully cathodic may be obtained by applying low voltage DC derived from normal AC mains supply.
This is achieved by transformer/rectifiers which supply DC to specially designed ‘inert’ anodes which will dissipate large currents without themselves suffering significant wastage. This approach is used in Impressed Current Systems.
Cathodic Protection Systems whether of the Sacrificial or Impressed Current type can be either used as the sole method of corrosion control, or in many instances, in conjunction with a coating.
Protection even with the best and most expensive coatings alone is seldom adequate. Coatings are always vulnerable to damage and can only be as good as atmospheric and surface conditions permit at the time of their application.
Cathodic Protection is therefore an essential ingredient in an overall corrosion control system for applications on ships as well as on off-shore structures, jetties, storage tanks and pipelines, both underground and sub-sea.
Cathodic protection systems can be designed either by experienced Wilson Taylor service engineers visiting a vessel or in the company’s design and estimating offices. When necessary, Classification Society approval can be obtained on an owner’s behalf.
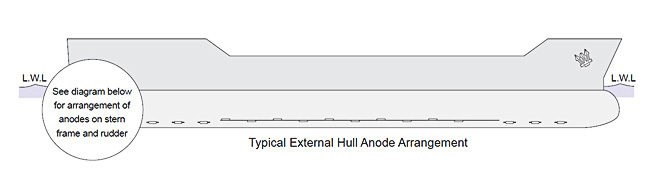
The number and type of sacrificial anodes required to protect the external hull of a specific vessel are calculated by taking into account several factors – size, the type of vessel, service conditions and the condition of the hull – whether it is new or in service.
Having determined the number of anodes required, it is important to ensure that the current distribution is effective and with the propeller located at the stern of the vessel – an area of major turbulence – it is necessary to fit a higher proportion of the anodes in the after part of the vessel, particularly close to the propeller.
The diagram below shows a typical anode distribution for a large vessel.
Anode Requirements For Full Hull Protection
Anode Requirements For Cargo and Ballast Tank Protection
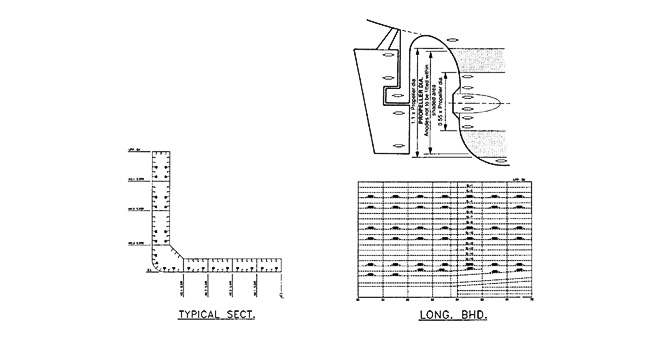
Plans showing anode layout, traced from owners' drawings, are provided free of charge when anodes are supplied. Bilge keels are not usually shown on a General Arrangement drawing so it is important that Wilson Taylor is always advised of the extent and location of the bilge keels.
Plans showing anode layout, traced from owners' drawings, are provided free of charge when anodes are supplied.

